Joint Replacement Application Example
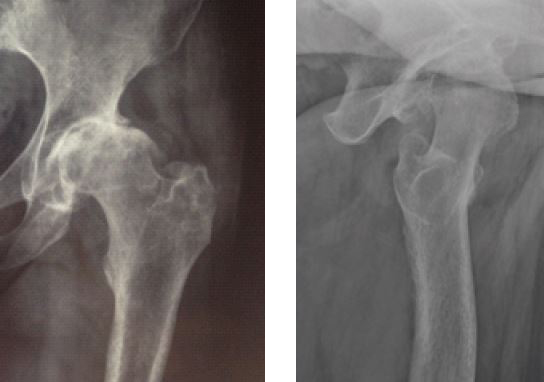
Results of prior hematogenous bacterial coxarthritis (Staph. aureus) in a 62-year-old woman.
Pre-operative X rays.
Anteroposterior and lateral views.
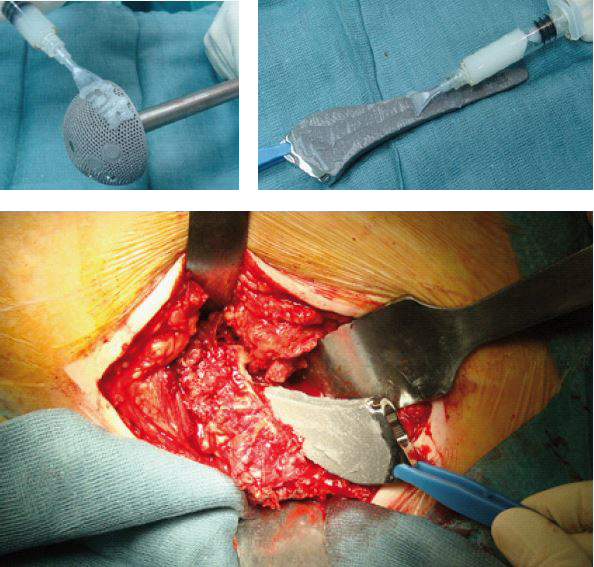
Cementless prosthetic components coated with DAC® hydrogel combined with 5% Vancomycin.
The DAC® coating is designed to resist during the introduction of a “press-fit” implant.
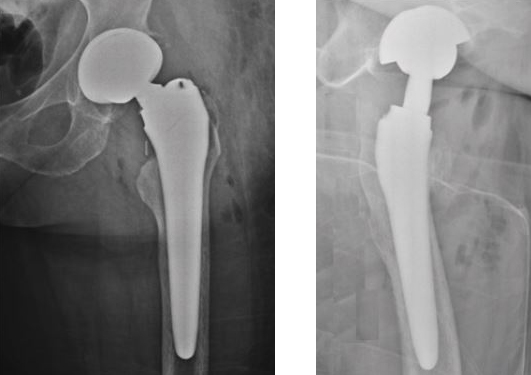
Insertion of the prosthetic components.
Post-operative X rays.
Control 3 months after surgery.
Radiolucent lines absent.
By kind permission of Prof. Carlo L. Romanò, Chief of the Reconstructive Surgery and Bone and Joint Infection Centre at the IRCCS Galeazzi Orthopaedic Institute, Milan.
Case Study – DAC® Hydrogel in One Stage Knee Revision
68 years old female obese patient who had previously a TKA on his right knee.
The first implant had to be revised due to pain, instability and implants aseptic loosening.
A first revision was performed employing a fully cemented hinged implant.
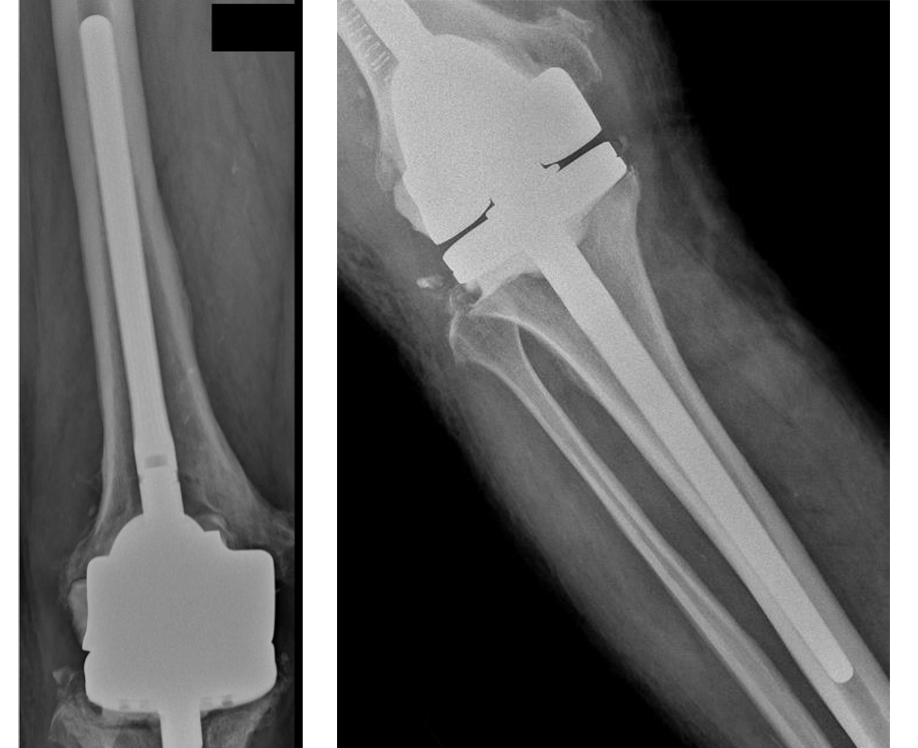
The patient post-op reported pain and swelling of the knee joint, with the X-Ray situation shown on the right.
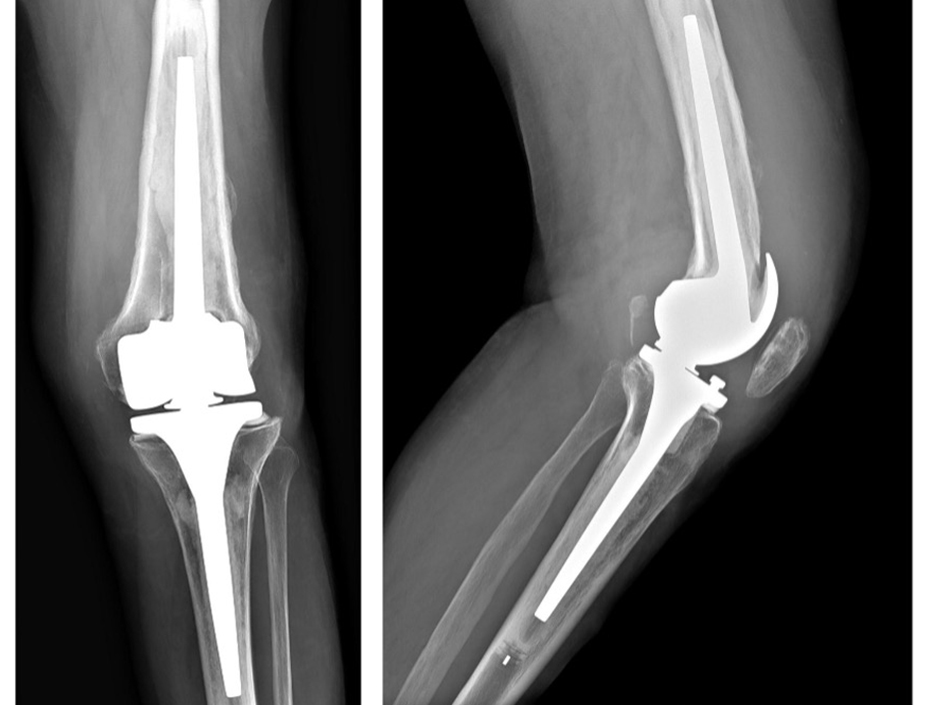
The patient had several risk factors such as obesity and peripheric vasculopathy. The knee joint was aspirated. The culture was found positive to Methicillin Resistant Staphylococcus Aureus. (MRSA).
The case was treated with a one stage revision employing a hinged implant with cementless intra medullary stems, performing an accurate joint debridement and cement removal.
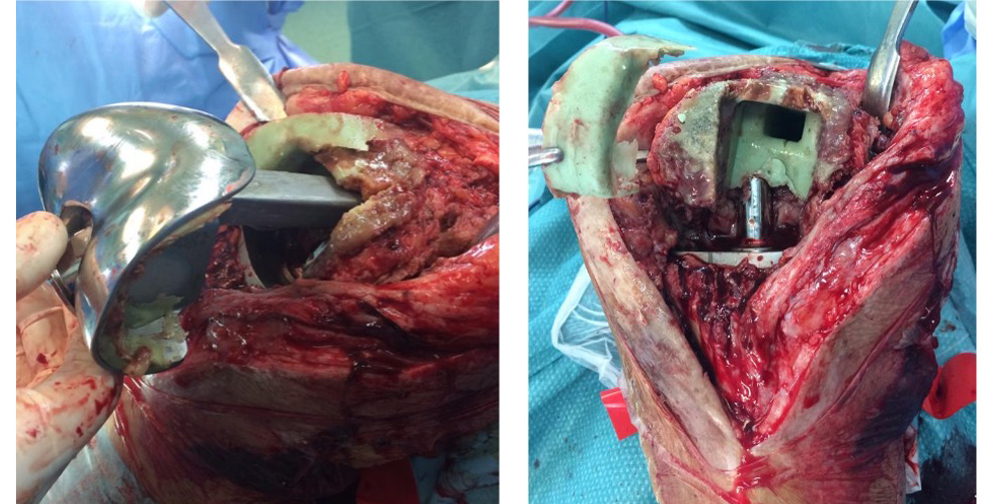
On the left intra-op pictures of the infected implant removal.
Before implantation, the prosthetic areas not in contact with cement were covered with DAC® Hydrogel. We used 10 ml hydrogel hydrated with a solution of water and 5% Vancomycin. The distal femoral and the proximal tibial prosthetic components were cemented using bone cement loaded with a combination of Vancomycin and Gentamycin. The DAC® hydrogel was spread on every implant component not in contact with cement including the IM stems, the poly insert and the articular surfaces.

On the left, the DAC® hydrogel spread on the prosthesis surface and the positioning of the implant.
The patient underwent an antibiotic therapy for 30 days following surgery with Meropenem (1gr. x 3) and Vancomycing (1gr. x 2).
Postoperative course went uneventfully with no signs of infection and a good healing of the surgical wound.
Post-Op Results
Postoperative course went uneventfully with no signs of infection and a good healing of the surgical wound.
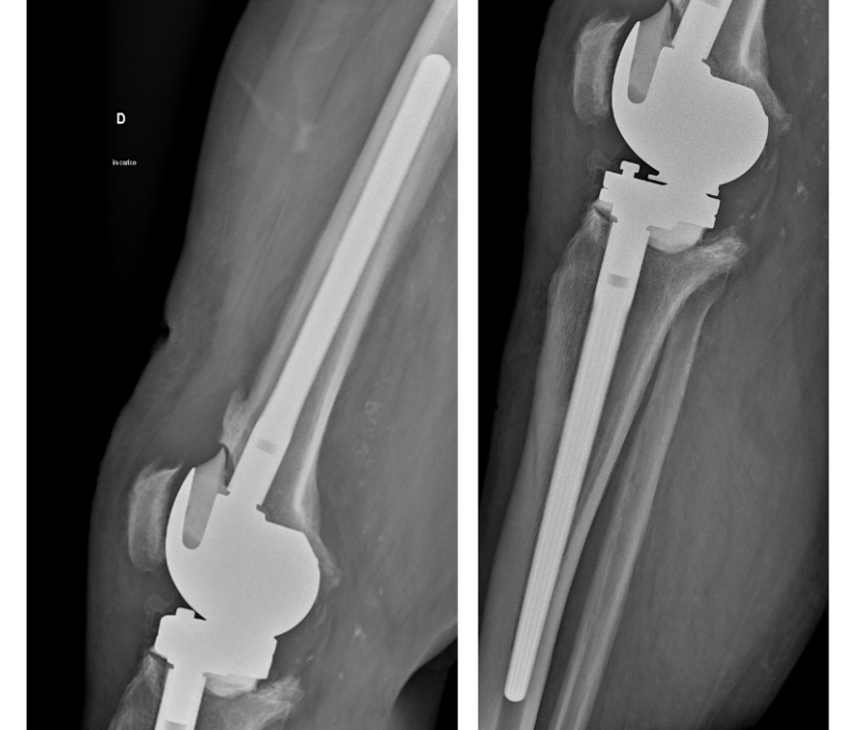
On the left, X-Ray Control 6 Months Post-Op with no signs of infection.
The patient showed a good functional recovery.
On the right, X-Ray Control 12 Months Post-Op.
The patient is infection free and she walks without aids.
Both Femoral and Tibial IM stems show a good bone contact and stability.